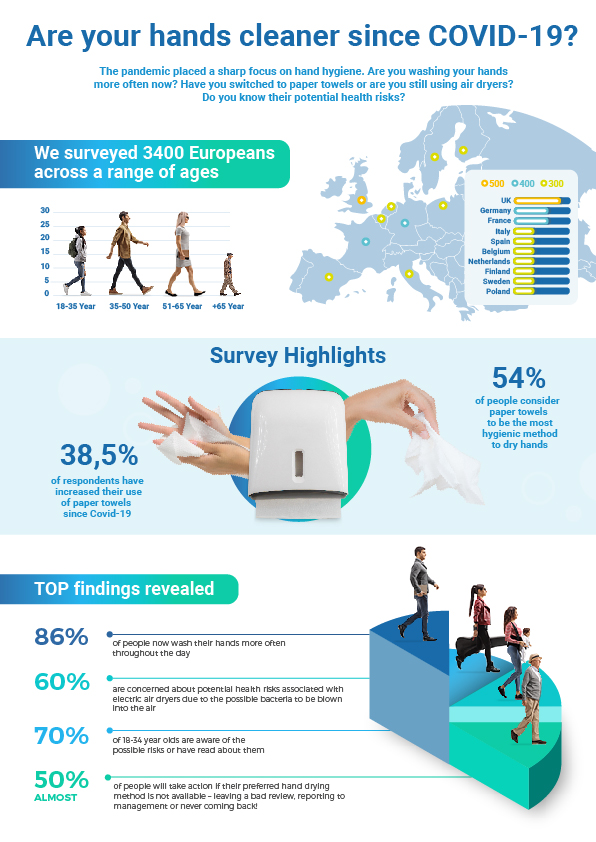
People wash hands more frequently, are concerned about health risks associated with electric dryers, and will take action if they don’t find their preferred drying option
Sidebar
Smart Health
Evonik launches non-animal-derived collagen platform Vecollan at commercial scale for medical device applications
Highly pure and reproducible; simplifies regulatory medical device approval
Improved performance due to highly tunable properties
Fermentation-based manufacturing leverages Evonik’s biotechnology platform

Evonik marks start of a revolution in cleaning and personal care with groundbreaking ceremony
Evonik is celebrating the start of construction of the world’s first commercial rhamnolipid production facility with a groundbreaking ceremony today.
First commercial rhamnolipids production site worldwide
Expands Evonik’s biotechnology platform
Portfolio transformation to sustainable solutions

Joint study by Messe Frankfurt and RWTH Aachen confirms safety of air quality in trade fair halls
Over the past two years, it has been virtually impossible to hold trade fairs, even though many exhibition halls in Germany have sophisticated ventilation systems in place. The health authorities operated on the assumption that, when people breathe, they emit potentially virus-infected aerosols in the same way they emit CO2 and that this would lead to widespread infections with SARS-CoV-2.
Fourth Industrial Revolution Technologies Can Play “Game-Changer” Role in Transforming Cancer Care
A new white paper published by the World Economic Forum, drafted with input from over 30 experts, analyses how Fourth Industrial Revolution Technologies, such as AI, blockchain and IoT, can deliver better cancer care.
The Oncology Data Model proposed by the white paper will be a “game changer” that captures data along the entire patient journey to transform every aspect of cancer care, from prevention and diagnosis to curative care and governance.

Definitive Agreement to Acquire Exelead will Strengthen Merck’s CDMO Offering for mRNA
Merck has signed a definitive agreement to acquire Exelead, a biopharmaceutical contract development and manufacturing organization (CDMO), for approximately USD 780 million in cash.

Taking hygiene measures to prevent hospital-acquired infections
A case study at the University Hospital Basel confirms the high effectiveness of an antimicrobial coating on adhesive films

MDI Health Launches Breakthrough Personalized AI Medication Platform with Potential to Save Millions of Lives
AI platform to also significantly reduce $528 billion of excess care costs in USA
New clinical study confirms platform’s transformative potential and early success
New $6M seed funding round to enable continued aggressive company growth

Fish, steak, roast and more: Plant-based and sustainable - meals out of the printer
"How? Printed food? That's way in the future, isn't the industry that far along yet..." Yes it is - the industry is even further ahead. Printed steaks, roasts, but also salmon and tuna are already on the supermarket shelves of various shops, also here in Austria. You don't believe that? Read here which products are already being additively manufactured from plant-based raw materials. By Sabine Slaughter

Upholding hygiene and safety in the HORECA sector post pandemic
By Fanis Papakostas, chairman, European Tissue Symposium
With bars and restaurants opening up across Europe, it feels like life is finally returning to some semblance of normality. The HORECA sector has been badly hit by the Covid-19 pandemic. It is now crucial to explore what needs to be done in order to restore the confidence of consumers and – to quote the recent G7 communique – “build back better”.

Top tips for a safe and healthy summer
Whether you are planning a vacation or a staycation, be sure to practice sound holiday hygiene

 Deutsch (Germany)
Deutsch (Germany)  Polski (PL)
Polski (PL) 






