ONCOVITA, a biotechnology company specializing in innovative vaccine development and a spin-off from the Institut Pasteur, and UNITHER Pharmaceuticals, a global leader in sterile unit-dose manufacturing, announce a strategic partnership to develop and produce a new combined prophylactic vaccine for the prevention of infectious diseases in children.
Sidebar
Smart Health
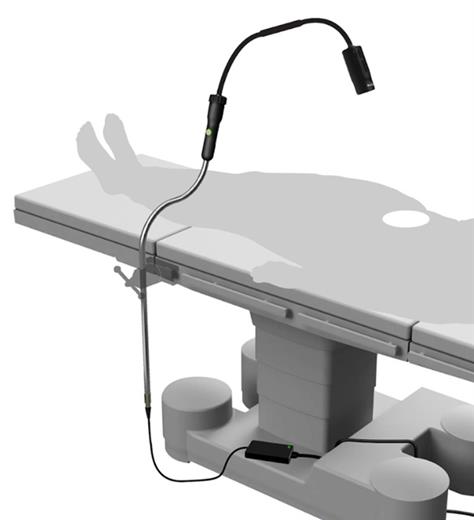
Syensqo partners with MezLight to launch the world’s first sterile reusable surgical task light
Novel illumination system with key components molded in Radel® PPSU for durability and repeated sterilization

Merck Partners with Opentrons Labworks, Inc., supporting Lab of the Future
Custom robotic workstation automates company’s broad portfolio of biology assays for academia, biotech, and pharma
Meets growing need for autonomous tools that boost throughput and reproducibility
Provides customers with verified and automated workflows
Merck, a leading science and technology company, and Opentrons Labworks, Inc., a leader in lab automation and accessible robotics, announced a multi-year agreement to automate assay kits on a custom Opentrons Flex® workstation.

Merck launches first cyber-physical trust platform to tackle issues of product safety
Merck launched M-Trust, a secure cyber-physical trust platform, addressing growing issues of product safety, traceability and counterfeiting.
M-Trust™ is a secure cyber-physical trust platform that enables the creation of digital twins for enhanced product quality and authenticity assurances
This cutting-edge new technology is being unveiled at CES 2025, the proving ground for breakthrough technologies
M-Trust™ platform is being launched in beta for immediate use by B2B users globally
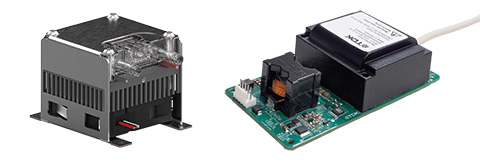
Reylon plasma presents innovative plasma devices at Arab Health 2025
Relyon plasma from Regensburg, a subsidiary of TDK Electronics, is presenting plasma devices for the healthcare sector at Arab Health. In addition to the MediPlas system for disinfection, the ViroCAP® system from cooperation partner Viromed Medical GmbH will also be presented for the first time.

Merck Invests €70 million to Expand ADC Manufacturing for Novel Cancer Therapies
Merck today announced a € 70 million expansion of its ADC manufacturing capabilities and capacity in St. Louis, Missouri, USA.
Triples manufacturing capacity to meet increased global demand for antibody-drug conjugates (ADCs)
Reinforces position as first commercially approved contract development and manufacturing organization (CDMO) for ADCs in North America
Expansion to create 170 jobs at Bioconjugation Center of Excellence in St. Louis, Missouri, USA

Why washrooms in French hospitals advise paper towels for drying hands
Blog by Professor Frédéric Barbut, medical microbiologist, Assistance Publique-Hôpitaux de Paris, hôpital Saint-Antoine, Paris
Did you know that we all carry many millions of bacteria on our hands? Anything between 100 to 1,000,000 per square centimetre of skin! But before you start to panic, rest assured that many of these bacteria occur naturally, along with fungi and viruses, and are normally harmless (or ‘not pathogenic’ to use the scientific term). They are known as ‘resident microflora’ and can actually be beneficial in protecting us from infection. Common strains include Staphylococcus and Micrococcus.

Merck Launches First Software for Complete Data Traceability in Microbial QC
M-Trace® Electronic Test Record Software offers digital cleanroom-friendly solution for sterility testing
Digitizes pharmaceutical microbial quality control workflows
Enables complete, real-time, comprehensive data traceability and regulatory compliance, reducing deviations and human error

Merck Signs MoU with KAIST to Advance Scientific Collaboration
Partnership to facilitate collaborative research in academia and industry to further progress in life sciences.
Partnership to facilitate collaborative research in academia and industry to further progress in life sciences
Multi-dimensional program to build local research capability and startup ecosystem as well as joint R&D projects
Further example of Merck’s close cooperation with Korea, following the recent € 300 million bioprocessing investment in Daejeon

eschbach Launches Shiftconnector Equipment Hub to Streamline Regulatory Compliance in the Pharmaceutical Industry
Global software developer eschbach, the provider of the Shiftconnector® enterprise manufacturing platform for the process industry, has a module, Shiftconnector® Equipment Hub. Designed for the pharmaceutical industry, the Equipment Hub ensures data integrity, simplifies search and retrieval across logs for audits and inspections, and streamlines compliance reporting.
Limula raises $6.8M to democratise access to life-saving Cell and Gene Therapies with Swiss-made manufacturing platform
Award-winning technology combines a bioreactor and a centrifuge into one single vessel for the first time, a new approach gentler to patient cells during process steps.

 Deutsch (Germany)
Deutsch (Germany)  Polski (PL)
Polski (PL) 



